| Mendeleev Communications |
|
|


|
|
|
This article is cited in 9 scientific papers (total in 9 papers) Communications Synthesis and properties of 1,3-diphenyl-5-(benzothiazol-2-yl)-6-R-verdazyls T. G. Fedorchenkoa, G. N. Lipunovaa, A. V. Shchepochkina, A. N. Tsmokalyukb, P. A. Slepukhinab, O. N. Chupakhinab a I.Ya. Postovsky Institute of Organic Synthesis, Ural Branch of the Russian Academy of Sciences, Ekaterinburg, Russian Federation b Institute of Chemical Engineering, Ural Federal University, Ekaterinburg, Russian Federation 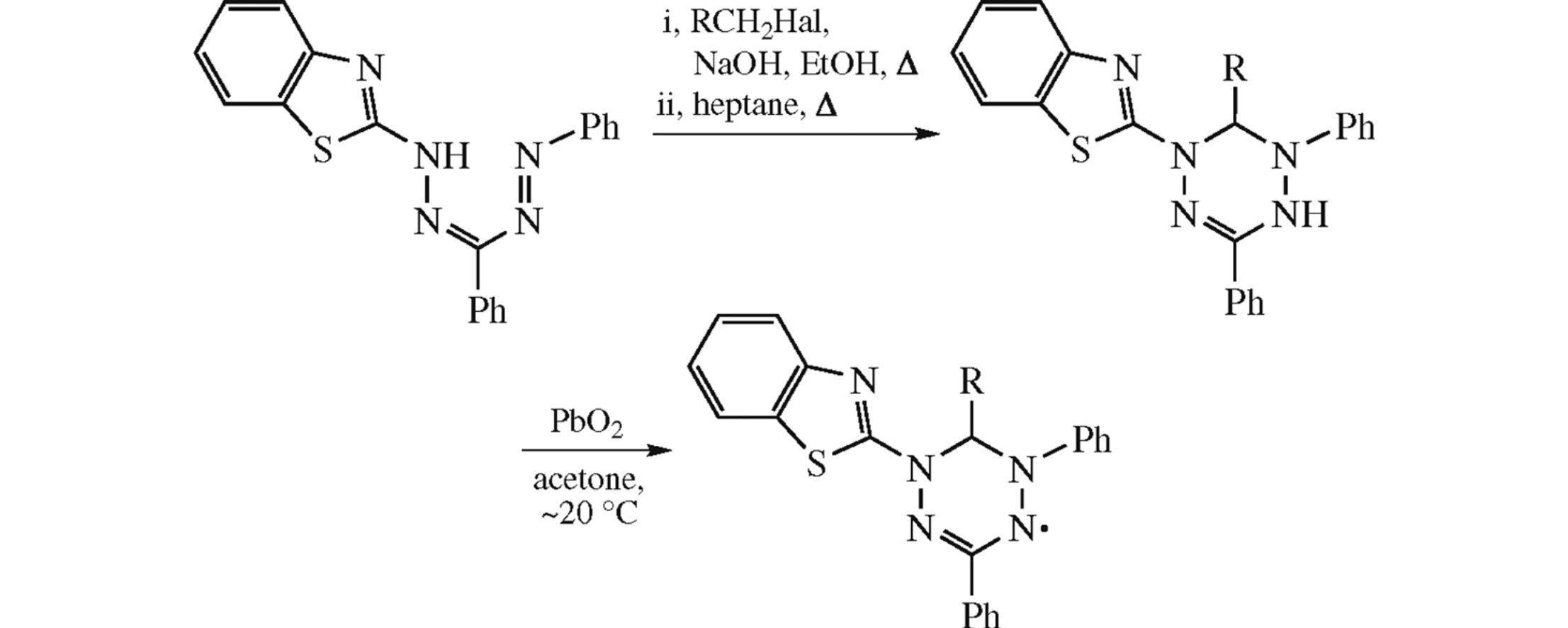
Citation: T. G. Fedorchenko, G. N. Lipunova, A. V. Shchepochkin, A. N. Tsmokalyuk, P. A. Slepukhin, O. N. Chupakhin, “Synthesis and properties of 1,3-diphenyl-5-(benzothiazol-2-yl)-6-R-verdazyls”, Mendeleev Commun., 28:3 (2018), |

|

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










