| Mendeleev Communications |
|
|


|
|
|
This article is cited in 12 scientific papers (total in 12 papers) Communications Synthesis and antihypotensive properties of 2-amino-2-thiazoline analogues with enhanced lipophilicity E. V. Nurievaa, T. P. Trofimovaab, A. A. Alexeeva, A. N. Proshinc, E. A. Chesnakovad, Yu. K. Grishina, K. A. Lyssenkoe, M. V. Filimonovad, S. O. Bachurinc, O. N. Zefirovaac a Department of Chemistry, M.V. Lomonosov Moscow State University, Moscow, Russian Federation b Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation c Institute of Physiologically Active Compounds, Federal Research Center of Problems of Chemical Physics and Medicinal Chemistry, Russian Academy of Sciences, Chernogolovka, Moscow Region, Russian Federation d Medical Radiology Research Center, Russian Academy of Medical Sciences, Obninsk, Kaluga Region, Russian Federation e A.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Moscow, Russian Federation 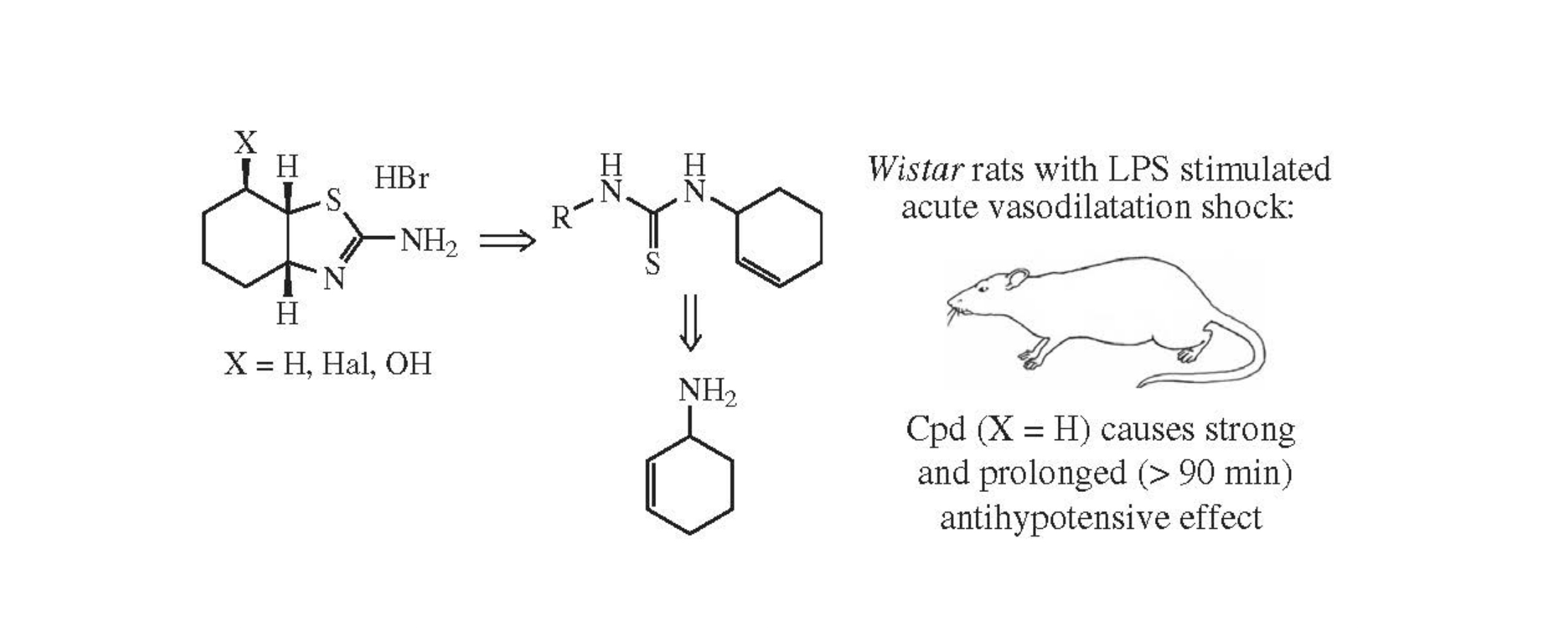
Citation: E. V. Nurieva, T. P. Trofimova, A. A. Alexeev, A. N. Proshin, E. A. Chesnakova, Yu. K. Grishin, K. A. Lyssenko, M. V. Filimonova, S. O. Bachurin, O. N. Zefirova, “Synthesis and antihypotensive properties of 2-amino-2-thiazoline analogues with enhanced lipophilicity”, Mendeleev Commun., 28:4 (2018), |

|
||||||||||||||||||||||||||||||||||

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










