| Mendeleev Communications |
|
|


|
|
|
This article is cited in 1 scientific paper (total in 1 paper) Communications Nickel-coordinated chiral enols and Michael addition intermediate stabilized by the Ni–C bond L. A. Hayriyana, A. F. Mkrtchyana, M. A. Moskalenkob, V. I. Maleevb, Z. T. Gugkaevab, M. M. Ilyinb, K. K. Babievskyb, P. V. Dorovatovskiic, V. N. Khrustalevcd, A. S. Peregudovb, Yu. N. Belokonb a Scientific and Production Center ‘Armbiotechnology’ of National Academy of Sciences of Republic Armenia, Yerevan, Armenia b A.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Moscow, Russian Federation c National Research Centre 'Kurchatov Institute', Moscow, Russian Federation d Peoples Friendship University of Russia (RUDN University), Moscow, Russian Federation 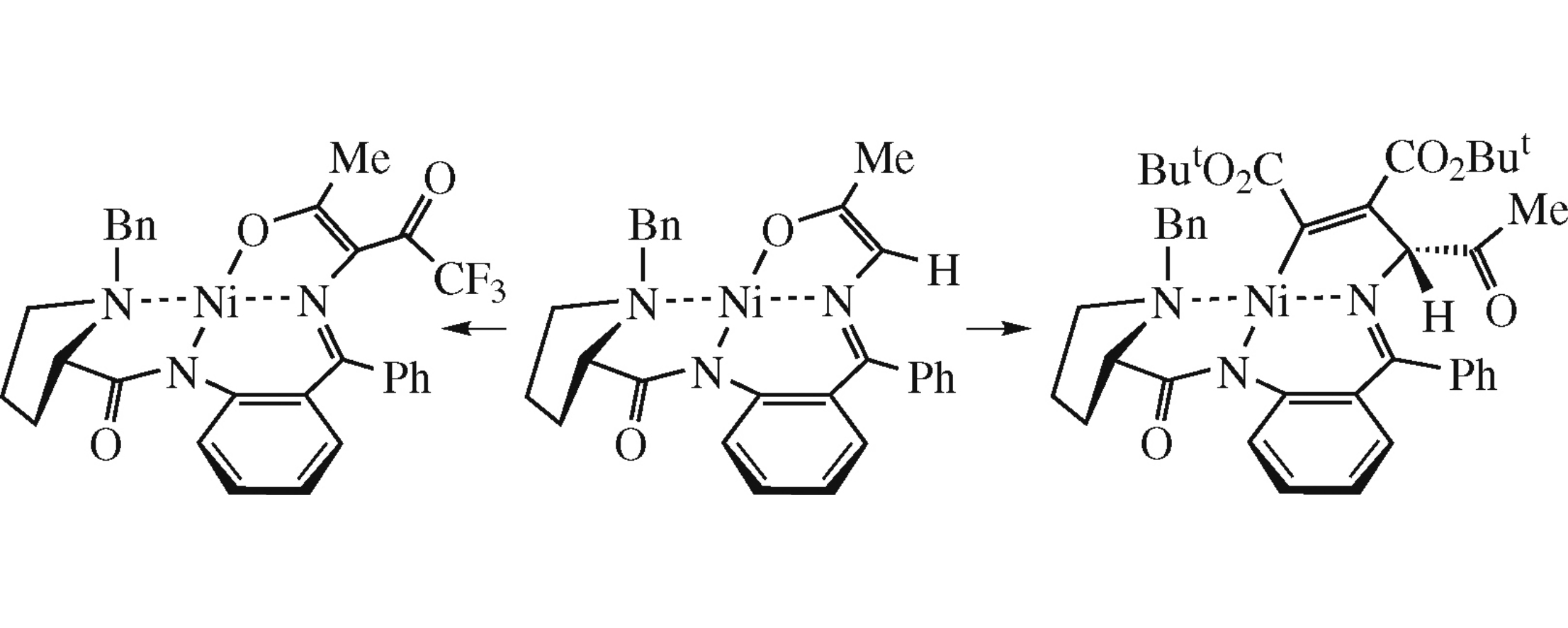
Citation: L. A. Hayriyan, A. F. Mkrtchyan, M. A. Moskalenko, V. I. Maleev, Z. T. Gugkaeva, M. M. Ilyin, K. K. Babievsky, P. V. Dorovatovskii, V. N. Khrustalev, A. S. Peregudov, Yu. N. Belokon, “Nickel-coordinated chiral enols and Michael addition intermediate stabilized by the Ni–C bond”, Mendeleev Commun., 28:5 (2018), |

|
||||||||||||||||||||||||||||||||||

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










