| Mendeleev Communications |
|
|


|
|
|
This article is cited in 4 scientific papers (total in 4 papers) Communications The C-3 chlorination of 2-aryl-1-hydroxyindoles Zh. V. Chirkovaa, M. V. Kabanovaa, S. I. Filimonova, I. G. Abramova, A. V. Sametb, G. A. Stashinab a Department of Organic Chemistry, Yaroslavl State Technical University, Yaroslavl, Russian Federation b N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russian Federation 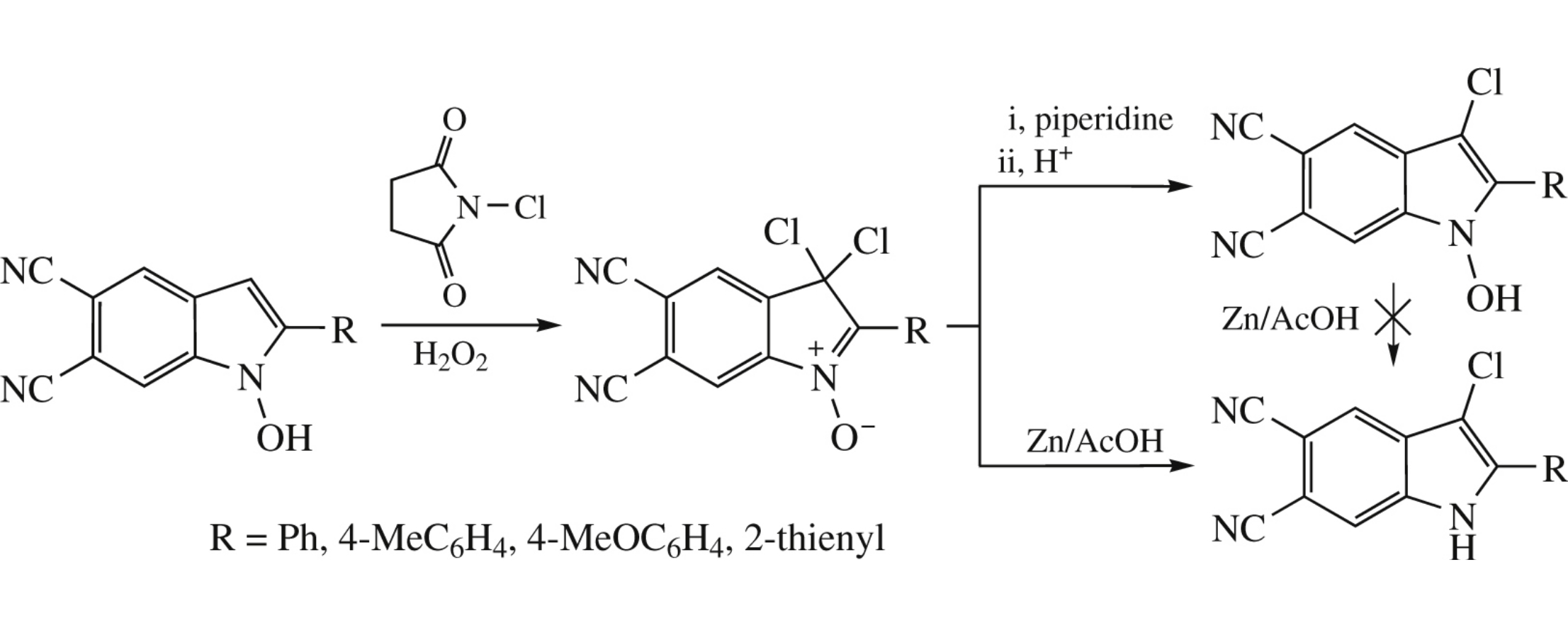
Citation: Zh. V. Chirkova, M. V. Kabanova, S. I. Filimonov, I. G. Abramov, A. V. Samet, G. A. Stashina, “The C-3 chlorination of 2-aryl-1-hydroxyindoles”, Mendeleev Commun., 27:5 (2017), |

|

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










