| Mendeleev Communications |
|
|


|
|
|
This article is cited in 6 scientific papers (total in 6 papers) Communications On the thermodynamic aspect of zinc oxide polymorphism: calorimetric study of metastable rock salt ZnO F. Yu. Sharikova, P. S. Sokolovb, A. N. Baranovc, V. L. Solozhenkob a St. Petersburg Mining University, St. Petersburg, Russian Federation b LSPM–CNRS, Université Paris Nord, Villetaneuse, France c Department of Chemistry, M.V. Lomonosov Moscow State University, Moscow, Russian Federation 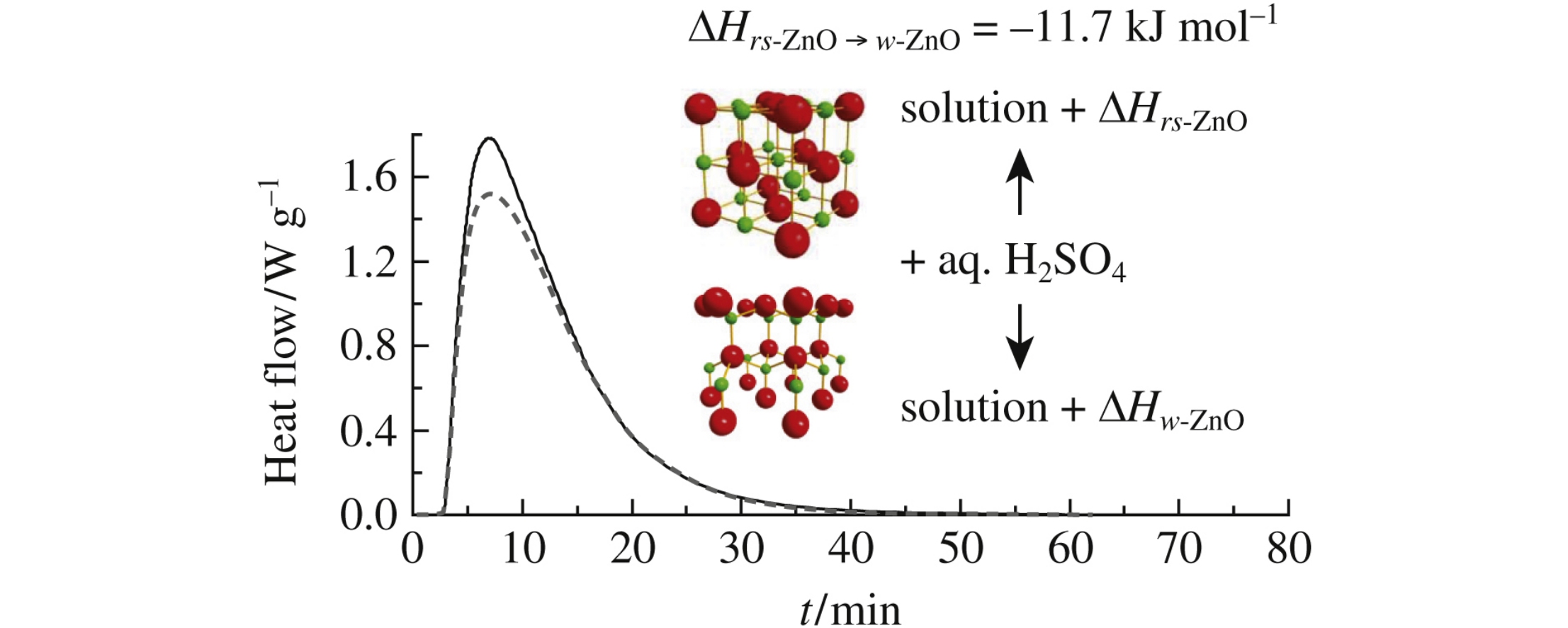
Citation: F. Yu. Sharikov, P. S. Sokolov, A. N. Baranov, V. L. Solozhenko, “On the thermodynamic aspect of zinc oxide polymorphism: calorimetric study of metastable rock salt ZnO”, Mendeleev Commun., 27:6 (2017), |

|

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










