| Mendeleev Communications |
|
|


|
|
|
This article is cited in 3 scientific papers (total in 3 papers) Communications A convenient synthesis of 8-hydroxy-1-tetralones from naphthalene-1,8-diol Zh. Zhuab, K. Yu. Koltunovac a Department of Natural Sciences, Novosibirsk State University, Novosibirsk, Russian Federation b Heilongjiang University, Harbin, P.R. China c G.K. Boreskov Institute of Catalysis, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russian Federation 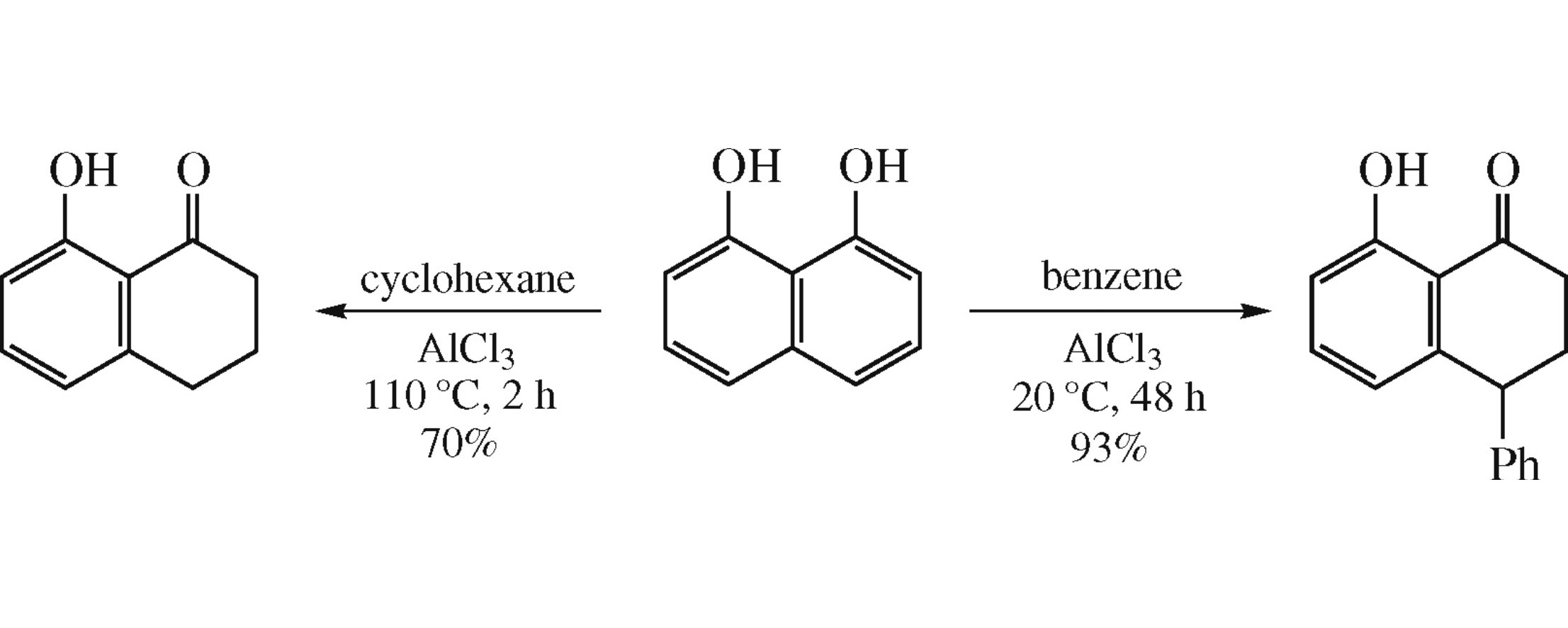
Citation: Zh. Zhu, K. Yu. Koltunov, “A convenient synthesis of 8-hydroxy-1-tetralones from naphthalene-1,8-diol”, Mendeleev Commun., 26:1 (2016), |

|
||||||||||||||||||||||||||||||||||

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










