| Mendeleev Communications |
|
|


|
|
|
This article is cited in 5 scientific papers (total in 5 papers) Communications Regioselective chelation in the reaction of N-trimethylsilyl-N-acetylglycine N’,N’-dimethylamide with chloro(chloromethyl)dimethylsilane S. Yu. Bylikinab, A. A. Korlyukovac, A. G. Shipova, D. E. Arkhipovac, N. A. Kalashnikovaa, V. V. Negrebetskya, Yu. I. Baukova a N.I. Pirogov Russian National Research Medical University, Moscow, Russian Federation b The Open University, Walton Hall, Milton Keynes, UK c A.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Moscow, Russian Federation 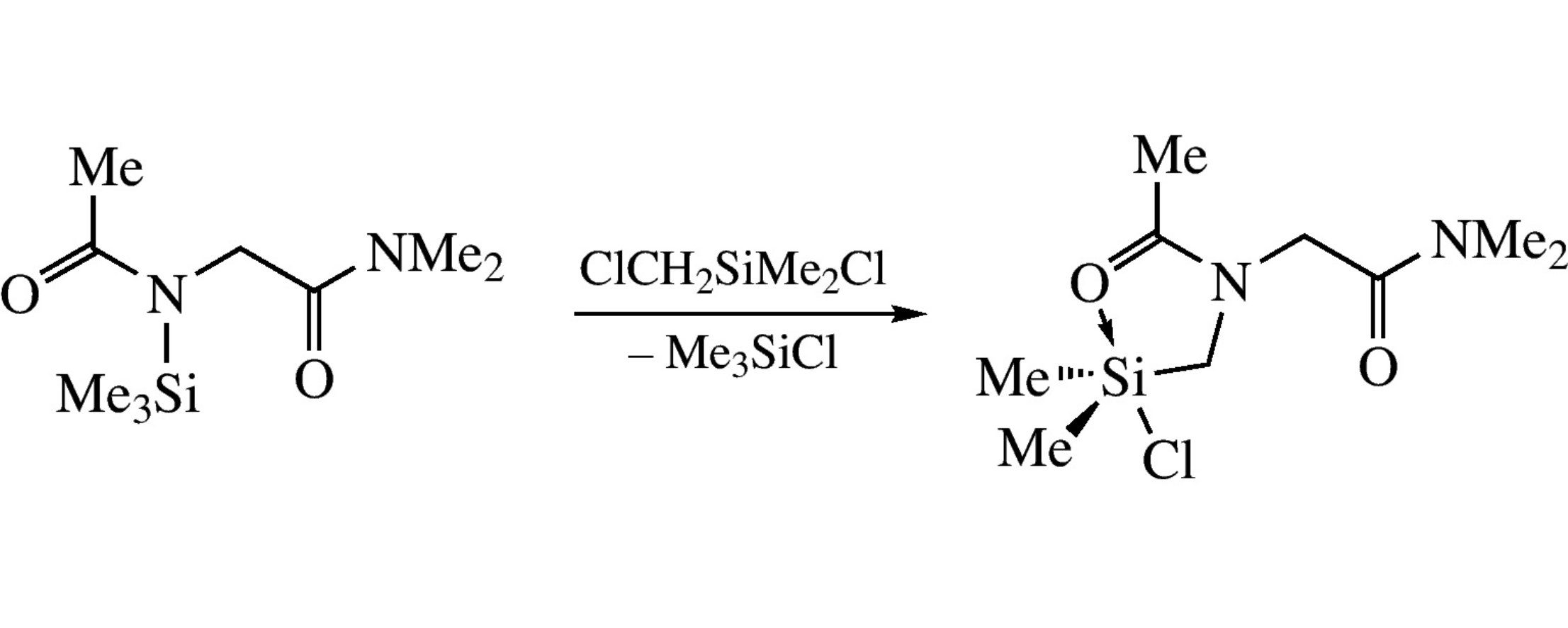
Citation: S. Yu. Bylikin, A. A. Korlyukov, A. G. Shipov, D. E. Arkhipov, N. A. Kalashnikova, V. V. Negrebetsky, Yu. I. Baukov, “Regioselective chelation in the reaction of N-trimethylsilyl-N-acetylglycine N’,N’-dimethylamide with chloro(chloromethyl)dimethylsilane”, Mendeleev Commun., 25:2 (2015), |

|
||||||||||||||||||||||||||||||||||

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










