| Mendeleev Communications |
|
|


|
|
|
This article is cited in 7 scientific papers (total in 7 papers) Communications Efficient synthesis of tertiary acyclic amides by the Chapman rearrangement of aryl benzimidates in ionic liquids M. A. Epishinaa, A. S. Kulikova, N. V. Ignat'evb, M. Schulteb, N. N. Makhovaa a N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russian Federation b Ionic Liquid Research Laboratory, Merck KGaA, Darmstadt, Germany 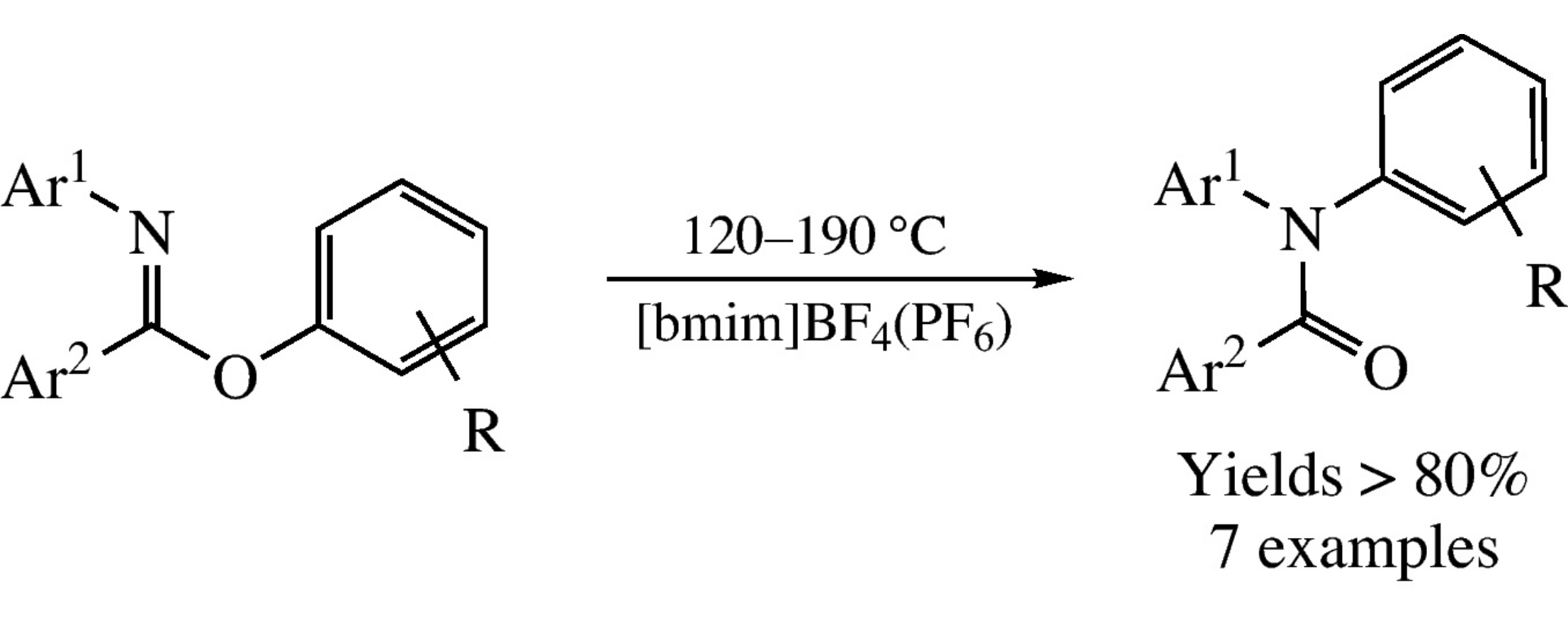
Citation: M. A. Epishina, A. S. Kulikov, N. V. Ignat'ev, M. Schulte, N. N. Makhova, “Efficient synthesis of tertiary acyclic amides by the Chapman rearrangement of aryl benzimidates in ionic liquids”, Mendeleev Commun., 25:2 (2015), |

|

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










