| Mendeleev Communications |
|
|


|
|
|
This article is cited in 1 scientific paper (total in 1 paper) Communications Synthesis of novel glutarimide derivatives via the Michael addition of (hetero)aromatic thiols: pronounced effect of sulfur oxidation on cytotoxicity towards multiple myeloma cell lines M. Adamchika, A. Bubyreva, D. Zhukovskya, P. Zhmurova, A. Bunevb, M. Krasavinac a Institute of Chemistry, St. Petersburg State University, St. Petersburg, Russian Federation b Medicinal Chemistry Centre, Togliatti State University, Togliatti, Russian Federation c Immanuel Kant Baltic Federal University, Kaliningrad, Russian Federation 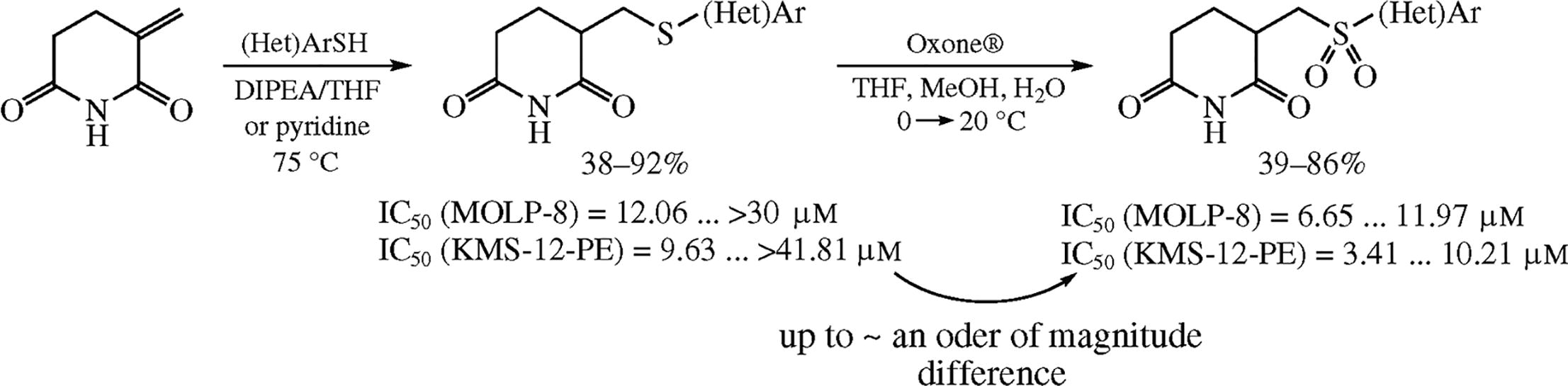
Citation: M. Adamchik, A. Bubyrev, D. Zhukovsky, P. Zhmurov, A. Bunev, M. Krasavin, “Synthesis of novel glutarimide derivatives via the Michael addition of (hetero)aromatic thiols: pronounced effect of sulfur oxidation on cytotoxicity towards multiple myeloma cell lines”, Mendeleev Commun., 33:1 (2023), |

|
||||||||||||||||||||||||||||||||||

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










