| Mendeleev Communications |
|
|


|
|
|
This article is cited in 10 scientific papers (total in 10 papers) Communications A novel urea derivative anticonvulsant: In vivo biological evaluation, radioreceptor analysis of GABAA receptors and molecular docking studies of enantiomers T. V. Shushpanovaa, N. A. Bokhanab, V. Yu. Kuksenokc, V. V. Shtrykovac, O. V. Shushpanovad, V. V. Udute a Mental Health Research Institute, Tomsk National Research Medical Center, Russian Academy of Sciences, Tomsk, Russian Federation b Siberian State Medical University, Tomsk, Russian Federation c National Research Tomsk Polytechnic University, Tomsk, Russian Federation d Scientific Center for Mental Health, Moscow, Russian Federation e E.D. Goldberg Scientific Research Institute of Pharmacology and Regenerative Medicine, Tomsk Research Center, Russian Academy of Sciences, Tomsk, Russian Federation 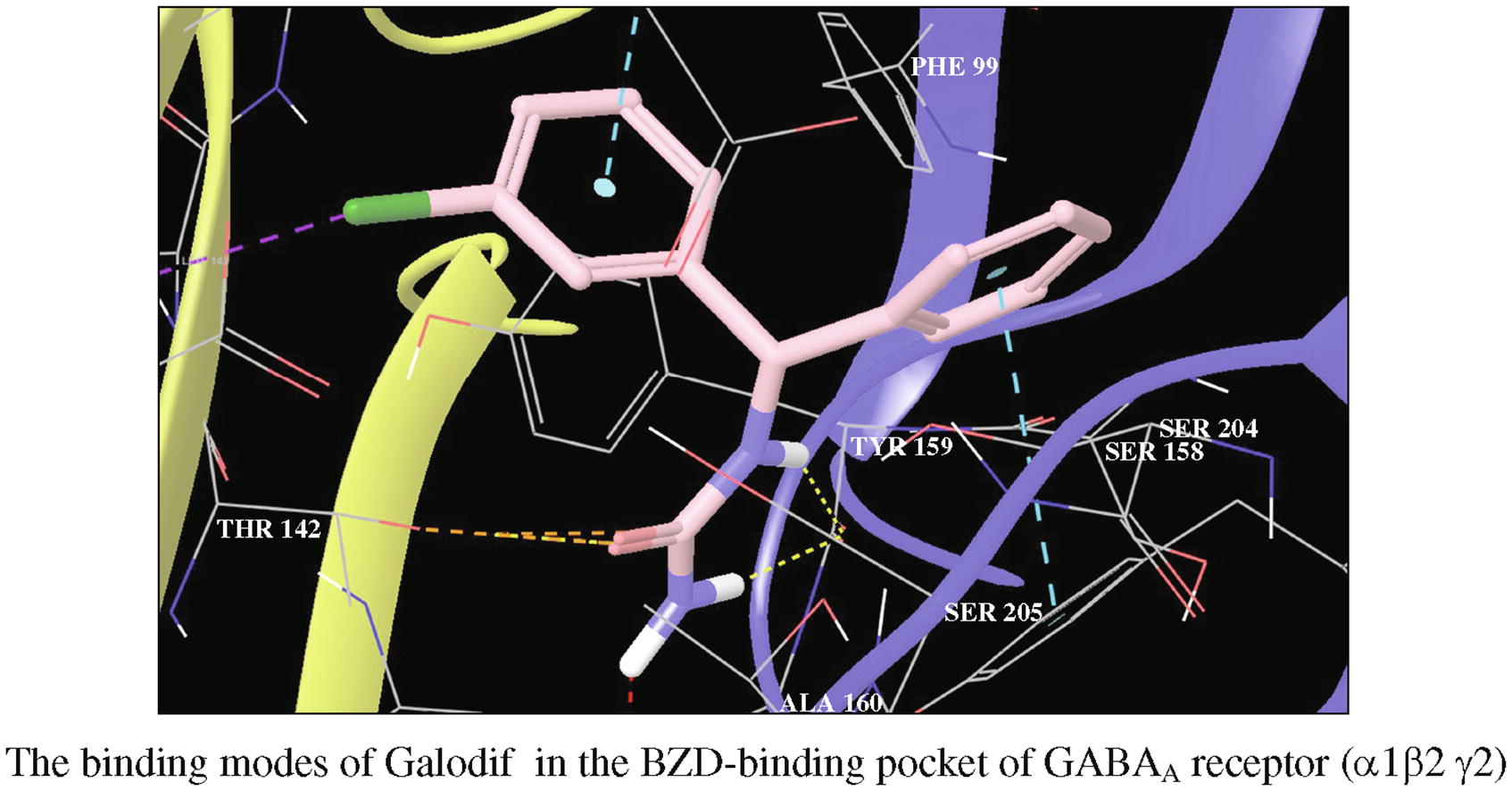
Citation: T. V. Shushpanova, N. A. Bokhan, V. Yu. Kuksenok, V. V. Shtrykova, O. V. Shushpanova, V. V. Udut, “A novel urea derivative anticonvulsant: In vivo biological evaluation, radioreceptor analysis of GABAA receptors and molecular docking studies of enantiomers”, Mendeleev Commun., 33:4 (2023), |

|

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










