| Mendeleev Communications |
|
|


|
|
|
This article is cited in 2 scientific papers (total in 2 papers) Communications Unexpected hydrazine- and hydroxylamine-induced transformations of aza-14-crown-4 incorporating 4-oxopiperidine-3-carboxylate moiety D. T. T. Nguyena, H. H. Truongb, N. T. Daoa, V. T. T. Trana, O. S. Gorchakovac, A. T. Lea a Faculty of Chemistry, University of Science, Vietnam National University, Hanoi, Hoan Kiem, Hanoi, Vietnam b Peoples Friendship University of Russia (RUDN University), Moscow, Russian Federation c 'Sanofi-aventis group' Representative office in Russia, Moscow, Russian Federation 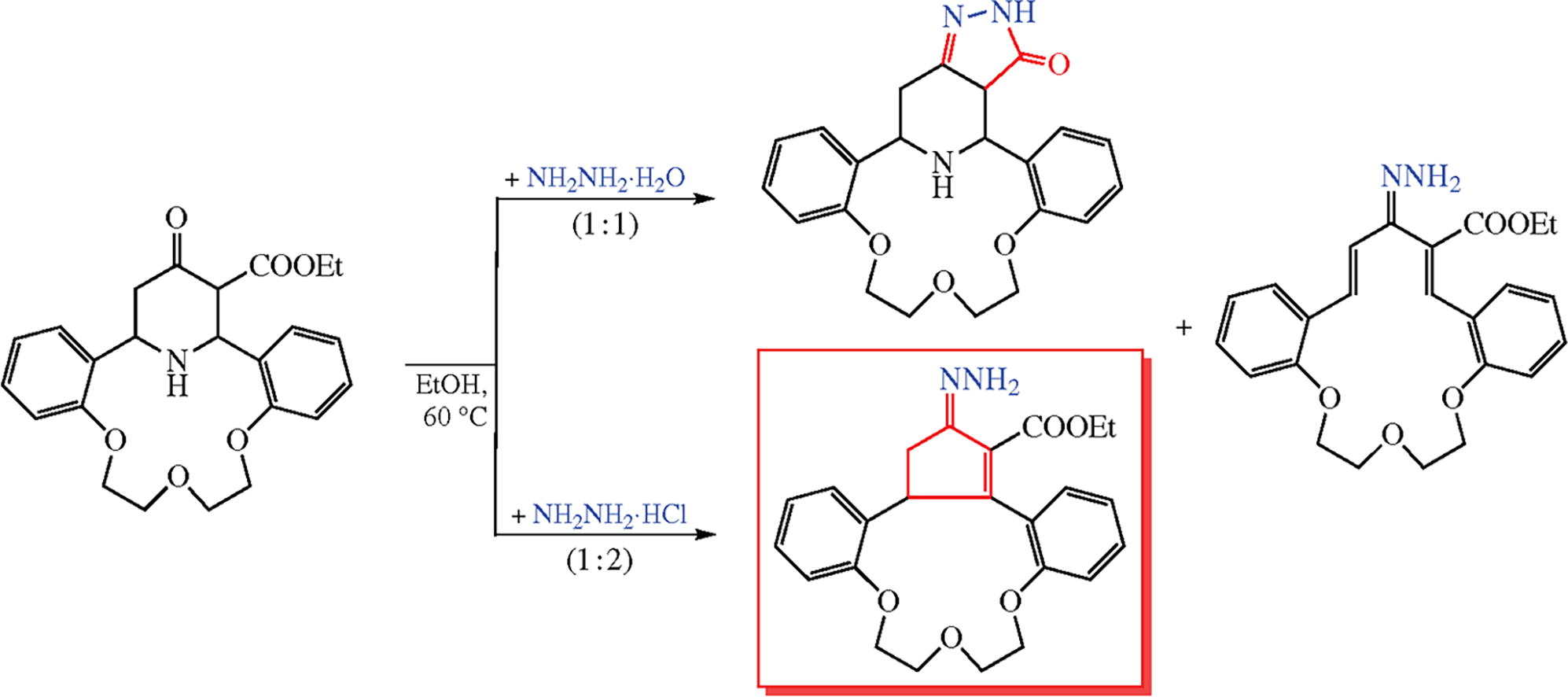
Citation: D. T. T. Nguyen, H. H. Truong, N. T. Dao, V. T. T. Tran, O. S. Gorchakova, A. T. Le, “Unexpected hydrazine- and hydroxylamine-induced transformations of aza-14-crown-4 incorporating 4-oxopiperidine-3-carboxylate moiety”, Mendeleev Commun., 33:5 (2023), |

|
||||||||||||||||||||||||||||||||||

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










