| Mendeleev Communications |
|
|


|
|
|
This article is cited in 3 scientific papers (total in 3 papers) Communications Effect of pressure on the low-temperature reaction of ethylene with N2O4 N. N. Tolkacheva, E. N. Khodota, I. I. Lischinerb, O. V. Malovab, V. A. Tartakovskya, V. Naudetc a N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russian Federation b Joint Institute for High Temperatures, Russian Academy of Sciences, Moscow, Russian Federation c Air Liquide, Campus Innovation Paris, Les Loges-en-Josas, France 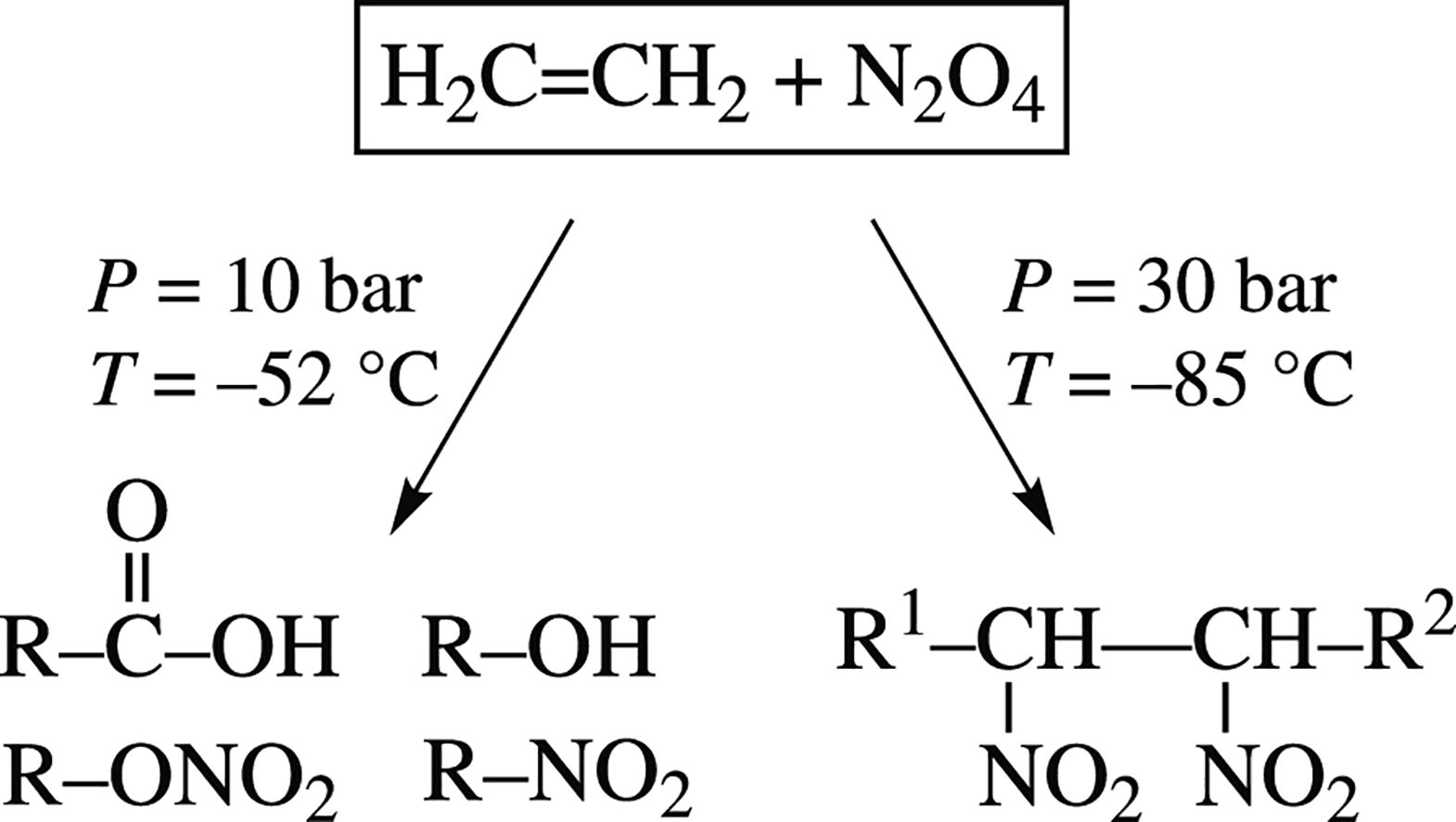
Citation: N. N. Tolkachev, E. N. Khodot, I. I. Lischiner, O. V. Malova, V. A. Tartakovsky, V. Naudet, “Effect of pressure on the low-temperature reaction of ethylene with N2O4”, Mendeleev Commun., 32:5 (2022), |

|
||||||||||||||||||||||||||||||||||

|
 Contact us:
Contact us: |
 Terms of Use Terms of Use
|
 Registration to the website Registration to the website |
 Logotypes Logotypes |
|










