| Mendeleev Communications |
|
|


|
|
|
Эта публикация цитируется в 20 научных статьях (всего в 20 статьях) Communications Intrinsic infrared absorption for carbon–fluorine bonding in fluorinated nanodiamond V. Yu. Osipovab, N. M. Romanovcd, K. Koganeb, H. Touharae, Y. Hattorie, K. Takaib a Ioffe Institute, St. Petersburg, Russian Federation b Department of Chemical Science and Technology, Hosei University, Tokyo, Japan c Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russian Federation d School of Engineering Science, LUT University, Lappeenranta, Finland e Faculty of Textile Science and Technology, Shinshu University, Ueda, Nagano, Japan 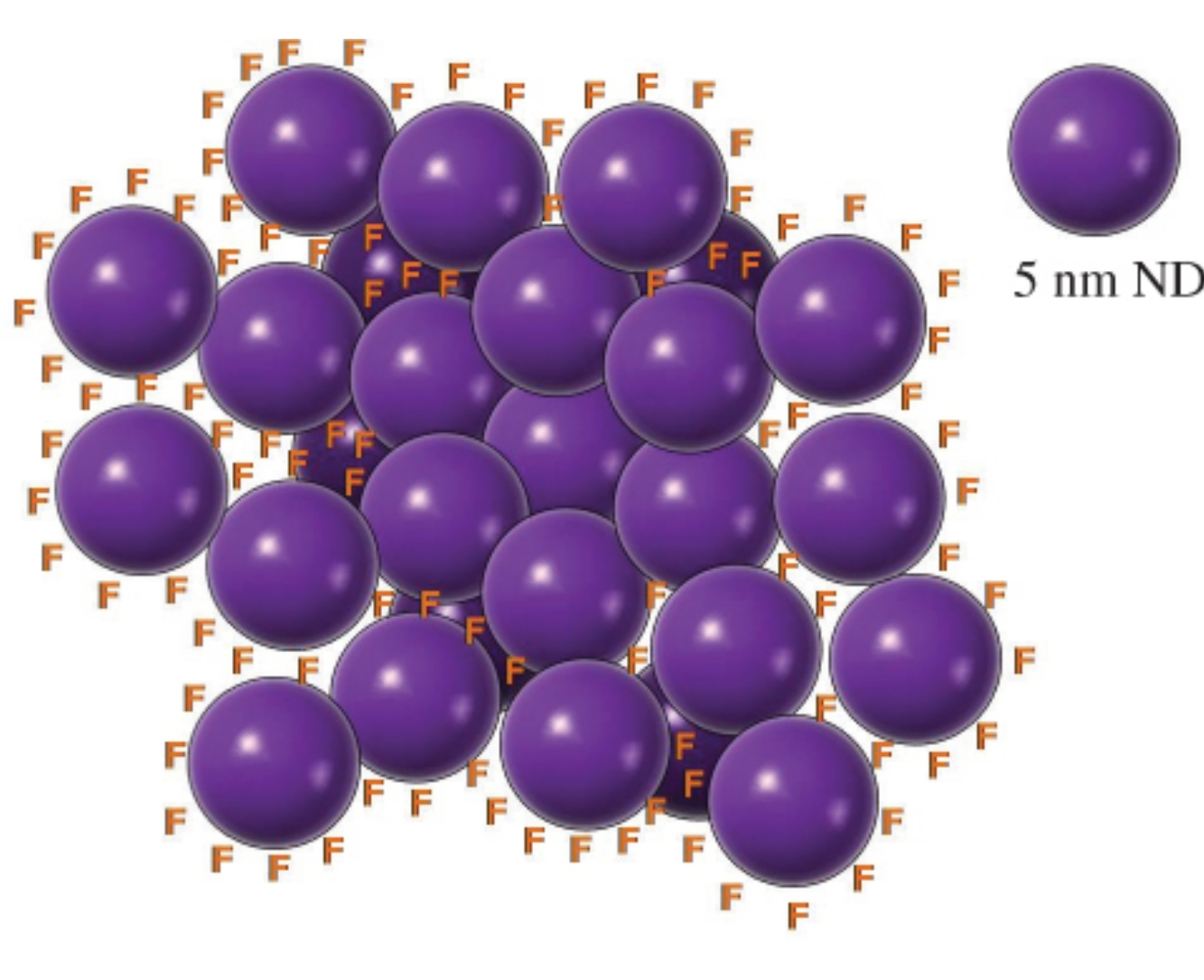
Образец цитирования: V. Yu. Osipov, N. M. Romanov, K. Kogane, H. Touhara, Y. Hattori, K. Takai, “Intrinsic infrared absorption for carbon–fluorine bonding in fluorinated nanodiamond”, Mendeleev Commun., 30:1 (2020), |

|

|
 Обратная связь:
Обратная связь: |
 Пользовательское соглашение Пользовательское соглашение
|
 Регистрация посетителей портала Регистрация посетителей портала |
 Логотипы Логотипы |
|










