| Mendeleev Communications |
|
|


|
|
|
Эта публикация цитируется в 5 научных статьях (всего в 5 статьях) Communications Modeling of the Diels–Alder reaction enantioselectivity by quantum mechanics and molecular mechanics A. A. Zeifmana, V. S. Stroylova, I. Yu. Titova, F. N. Novikova, O. V. Stroganova, I. Svitankoab, G. G. Chilova a N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, Russian Federation b Department of Chemistry, M.V. Lomonosov Moscow State University, Moscow, Russian Federation 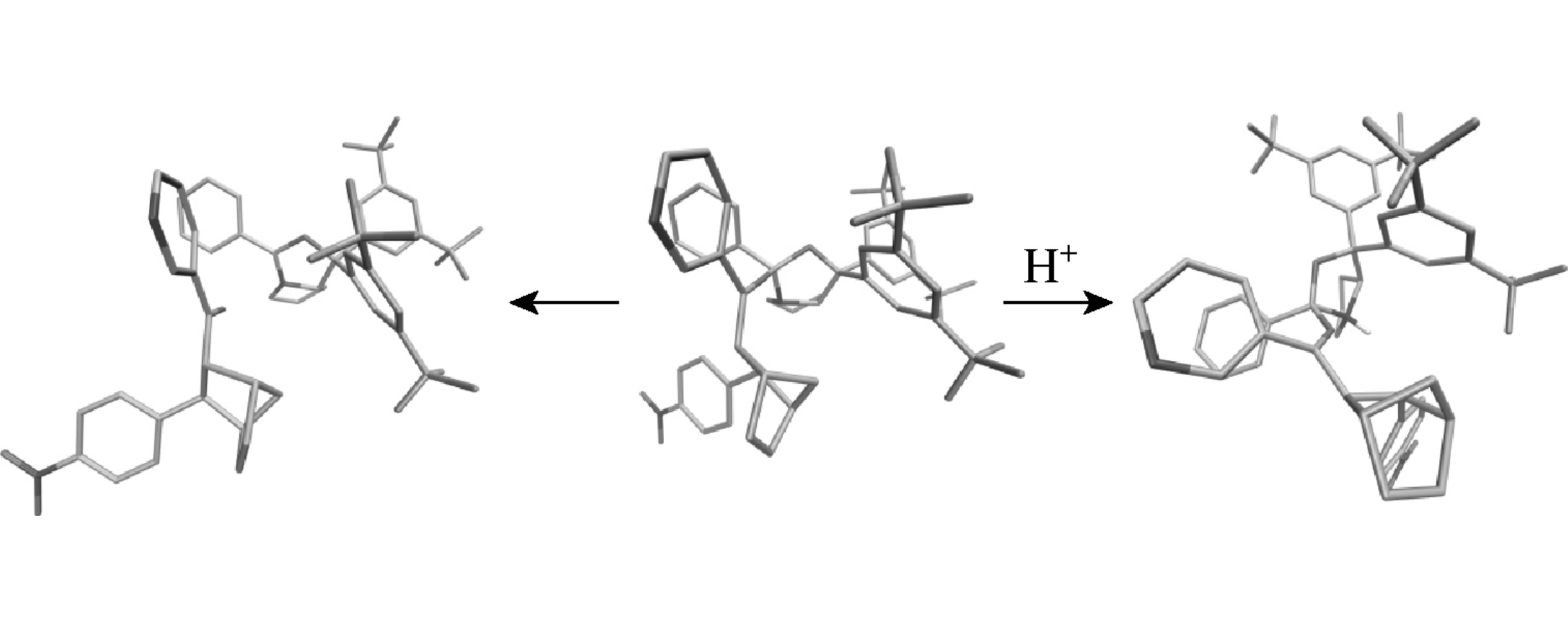
Образец цитирования: A. A. Zeifman, V. S. Stroylov, I. Yu. Titov, F. N. Novikov, O. V. Stroganov, I. Svitanko, G. G. Chilov, “Modeling of the Diels–Alder reaction enantioselectivity by quantum mechanics and molecular mechanics”, Mendeleev Commun., 25:4 (2015), |

|

|
 Обратная связь:
Обратная связь: |
 Пользовательское соглашение Пользовательское соглашение
|
 Регистрация посетителей портала Регистрация посетителей портала |
 Логотипы Логотипы |
|










