| Mendeleev Communications |
|
|


|
|
|
Эта публикация цитируется в 9 научных статьях (всего в 9 статьях) Communications Reaction of α,β-alkynylketones with β-amino alcohols: pseudoephedrine- assisted cleavage of triple bond via formal internal redox process S. F. Vasilevskiiab, M. P. Davydovaa, V. I. Mamatyukbc, N. V. Pleshkovac, D. S. Fadeevc, I. V. Alabugind a V.V. Voevodsky Institute of Chemical Kinetics and Combustion, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russian Federation b Novosibirsk State University, Novosibirsk, Russian Federation c N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russian Federation d Department of Chemistry, Florida State University, Tallahassee, USA 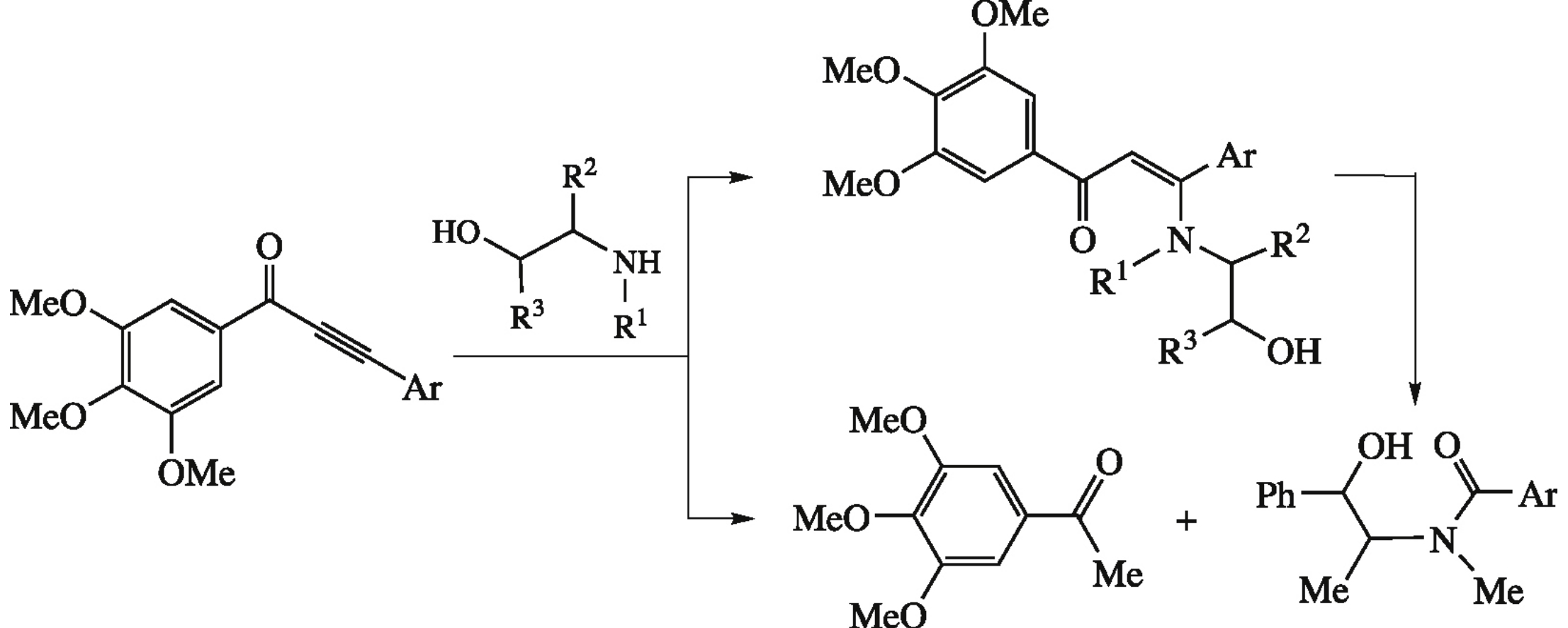
Образец цитирования: S. F. Vasilevskii, M. P. Davydova, V. I. Mamatyuk, N. V. Pleshkova, D. S. Fadeev, I. V. Alabugin, “Reaction of α,β-alkynylketones with β-amino alcohols: pseudoephedrine- assisted cleavage of triple bond via formal internal redox process”, Mendeleev Commun., 25:5 (2015), |

|
||||||||||||||||||||||||||||||||||||||||||

|
 Обратная связь:
Обратная связь: |
 Пользовательское соглашение Пользовательское соглашение
|
 Регистрация посетителей портала Регистрация посетителей портала |
 Логотипы Логотипы |
|










