| Mendeleev Communications |
|
|


|
|
|
Эта публикация цитируется в 3 научных статьях (всего в 3 статьях) Communications TADDOL-based P,S-bidentate diastereomeric ligands in asymmetric allylation and hydrogenation reactions K. N. Gavrilova, I. V. Chuchelkina, A. A. Shiryaevbc, I. D. Firsina, V. M. Truninaa, V. K. Gavrilova, Ya. P. Bityakd, D. A. Fedorovd, V. S. Zimarevae, N. S. Goulioukinaaef a Department of Chemistry, S.A. Esenin Ryazan State University, Ryazan, Russian Federation b I.P. Pavlov Ryazan State Medical University, Ryazan, Russian Federation c Scientific, Educational and Innovation Center for Chemical and Pharmaceutical Technologies, B.N. Yeltsin Ural Federal University, Ekaterinburg, Russian Federation d Moscow Institute of Physics and Technology (National Research University), Dolgoprudny, Moscow Region, Russian Federation e Department of Chemistry, M.V. Lomonosov Moscow State University, Moscow, Russian Federation f A.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russian Federation 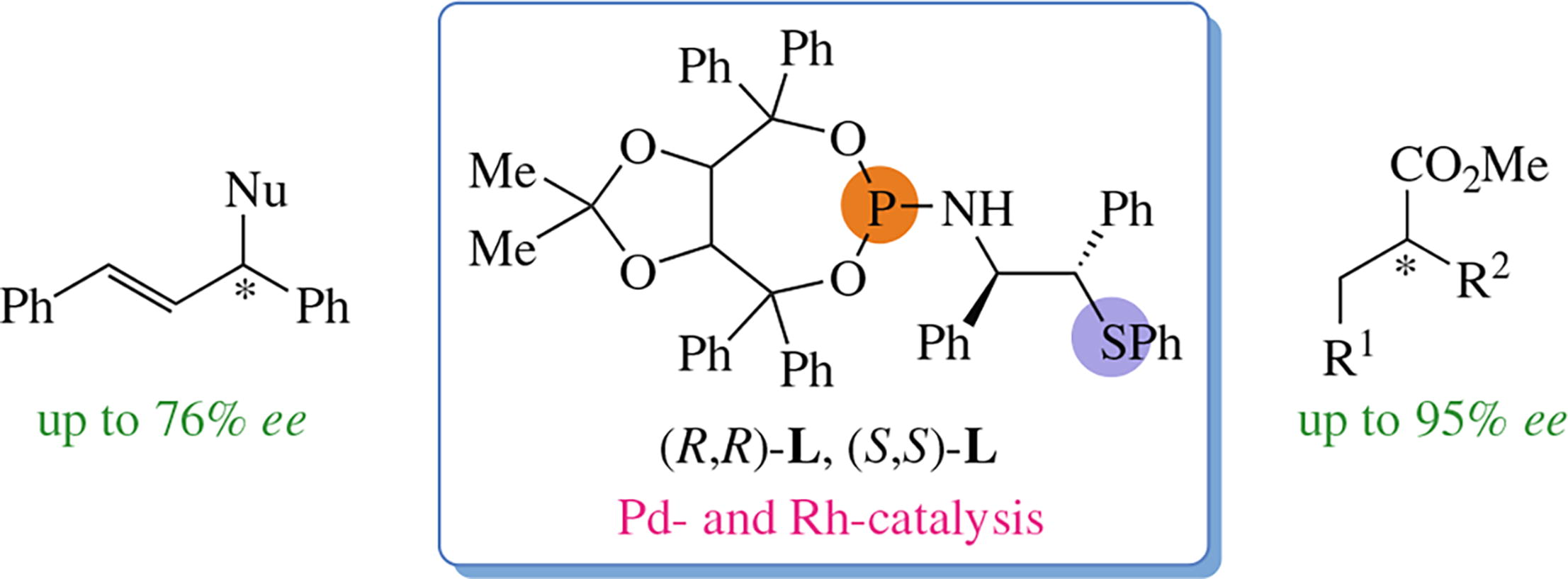
Образец цитирования: K. N. Gavrilov, I. V. Chuchelkin, A. A. Shiryaev, I. D. Firsin, V. M. Trunina, V. K. Gavrilov, Ya. P. Bityak, D. A. Fedorov, V. S. Zimarev, N. S. Goulioukina, “TADDOL-based P,S-bidentate diastereomeric ligands in asymmetric allylation and hydrogenation reactions”, Mendeleev Commun., 33:6 (2023), |

|
||||||||||||||||||||||||||||||||||||

|
 Обратная связь:
Обратная связь: |
 Пользовательское соглашение Пользовательское соглашение
|
 Регистрация посетителей портала Регистрация посетителей портала |
 Логотипы Логотипы |
|










