| Mendeleev Communications |
|
|


|
|
|
Эта публикация цитируется в 9 научных статьях (всего в 9 статьях) Communications Stereoselective arylthiolation of dehydroalanine in the NiII coordination environment: the stereoinductor of choice O. A. Levitskiya, O. I. Aglamazovaa, A. V. Dmitrievaa, V. A. Soloshonokbc, H. Moriwakid, Yu. K. Grishina, T. V. Magdesievaa a Department of Chemistry, M.V. Lomonosov Moscow State University, Moscow, Russian Federation b IKERBASQUE, Basque Foundation for Science, Bilbao, Spain c Department of Organic Chemistry I, Faculty of Chemistry, University of Basque Country UPV/EHU, San Sebastian, Spain d Hamari Chemical Ltd, Osaka, Japan 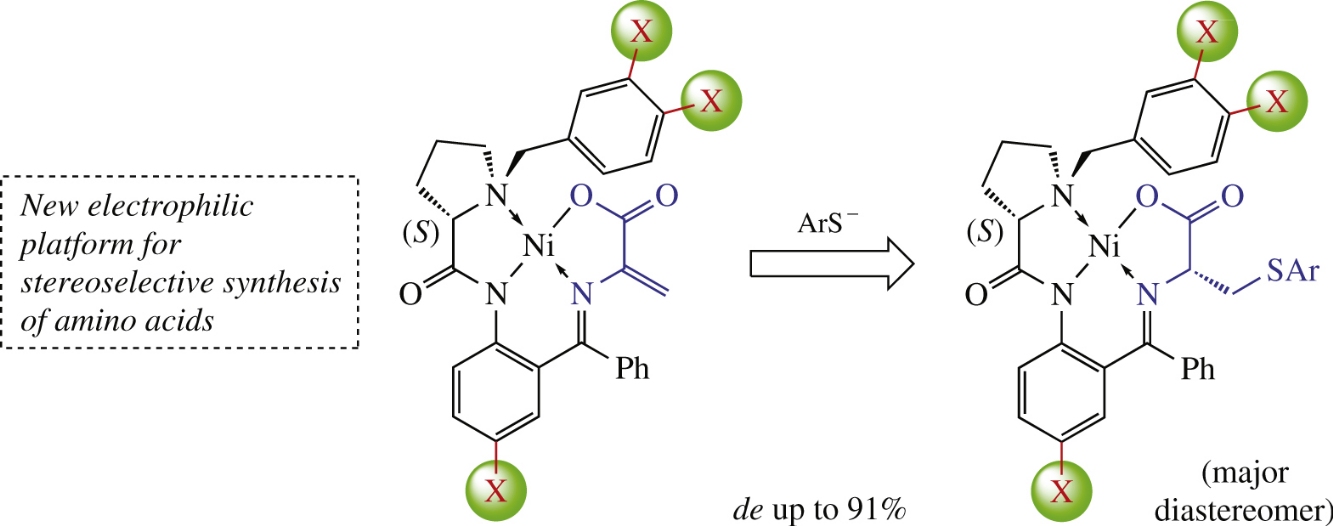
Образец цитирования: O. A. Levitskiy, O. I. Aglamazova, A. V. Dmitrieva, V. A. Soloshonok, H. Moriwaki, Yu. K. Grishin, T. V. Magdesieva, “Stereoselective arylthiolation of dehydroalanine in the NiII coordination environment: the stereoinductor of choice”, Mendeleev Commun., 31:3 (2021), |

|
||||||||||||||||||||||||||||||||||||

|
 Обратная связь:
Обратная связь: |
 Пользовательское соглашение Пользовательское соглашение
|
 Регистрация посетителей портала Регистрация посетителей портала |
 Логотипы Логотипы |
|










